ADAM: Non-Hormonal Male Birth Control Gel Enters Promising Phase 2 Trials in Australia
A potential game-changer in male contraception is now in Phase 2 clinical trials. Developed by U.S. biotech company Contraline, ADAM is a non-hormonal, injectable contraceptive gel that works by physically blocking the release of sperm—offering men a new, temporary birth control option without altering hormone levels.
💉 How ADAM Works
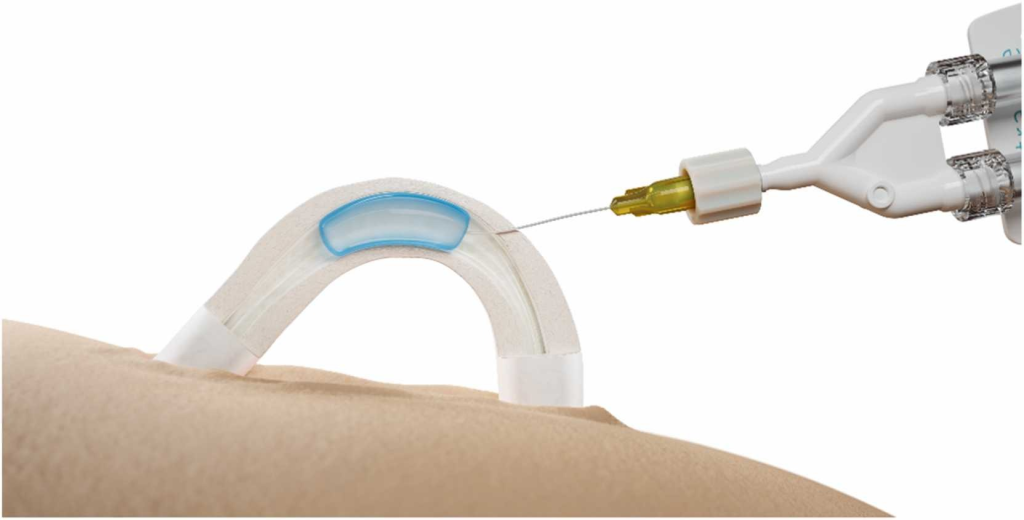
The procedure is quick—just 10 minutes. A doctor injects a soft hydrogel into the vas deferens, a duct that transports sperm. This gel forms a physical barrier, preventing sperm from being released during ejaculation.
Unlike previous male contraceptive methods that rely on hormone regulation—often leading to mood changes and reduced libido—ADAM’s non-hormonal approach is designed to avoid these side effects.
📊 What Early Trials Show
In Phase 1 trials, ADAM significantly reduced sperm count with minimal reported side effects. Most men resumed normal activities shortly after the procedure. Reported short-term effects include:
- Mild pain or discomfort at the injection site
- Temporary swelling
- Possible partial blockage or gel migration
While these effects were rare, researchers stress that long-term safety and full reversibility are still under evaluation.
🧬 Why ADAM Stands Out
Most male birth control research has focused on hormone-based solutions, which often come with unwanted side effects like:
- Fatigue
- Mood changes
- Reduced sexual drive
ADAM bypasses these issues by focusing solely on mechanical contraception, offering a safer, more targeted solution—at least in theory.
🧪 The Phase 2 Trial in Australia
Contraline’s Phase 2 trial is currently underway in multiple Australian cities. It includes volunteers aged 25 to 40, each of whom will be monitored for up to two years.
Researchers aim to track:
- Sperm suppression effectiveness
- Health impacts over time
- Ease of reversibility
Dr. Amanda Lewis, a reproductive health researcher based in Melbourne, says:
“This trial offers an exciting opportunity to expand contraceptive choices. Still, we need robust data before making any clinical recommendations.”
⏳ When Will ADAM Be Available?
As of now, ADAM is not approved for use anywhere in the world. If Phase 2 proves successful, further trials in the U.S., Europe, and other countries will be necessary before seeking regulatory approval.
Final trial results are expected by late 2026, meaning widespread access is still several years away.
🌐 Public Reaction
Public response has been mixed. Many see ADAM as a long-overdue innovation in male birth control. Others voice concerns about safety, reversibility, and the unknowns that come with any new medical product.
Conclusion:
While ADAM is still in the research phase, it represents a promising shift in male reproductive health. If proven safe and effective, this injectable gel could redefine contraceptive responsibility—sharing the burden more equally between men and women.